Those
who read my previous blog will have been introduced to viral restriction
factors and will, hopefully, remember that my inspiration for writing on such a
topic was the discovery of a new member of these proteins; human schlafen 11.
Those who didn’t read the previous post may find it useful to go back so as to aid
in the understanding of this post.
Besides the discovery of a new restriction factor, this paper caught my
eye as a wonderful demonstration of how logical and step-by-step science can be.
Hopefully I will be able to convey this logical progression through this blog
post, as it really is a very nicely written paper.
As
I mentioned in the previous post, the paper in question is from Michael David’s
lab at University of California, San Diego and has now been published in theprint issue of the journal Nature, as of the 1st November. The
paper centres on the schlafen genes (SLFN), of which there are six in humans. These
proteins are of particular interest since they have been consistently shown to
be highly expressed in cells and organisms that are infected with viruses or
bacteria, yet they had no known function. Along with the data from in vivo studies (in living organisms
such as mice), the team also looked at cell lines (collections of cells grown
in a lab) and found that there was differential expression between two very
closely related cell lines, HEK293 and HEK293T cells. They found that HEK293
cells (hereafter referred to simply as 293 cells) express high levels of SLFN5
and SLFN11, while the HEK293T cells (293T cells) did not. Finding this
different level of expression gives the team two cell lines that are easy to
conduct experiments in (unlike animals) and allows for easy comparison of the
situation with and without the SLFN proteins.
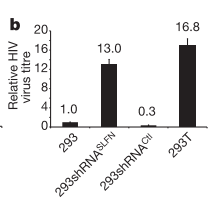
The
first experiment the team conducted was to look at the effect of removal of the
SLFN genes from 293 cells (the cells that express) and compare this with the
293T cells and normal 293 cells. Any new similarities seen between the 293T
cells and the 293 cells that have had the SLFN genes removed may be due to the
lack of SLFN genes. If these similarities are different to normal 293 cells
then it is even more likely to be due to the lack of SLFN genes (sorry if that
is hard to follow with the similar nomenclature). The team infected the cells
with multiple viruses including a HIV virus that causes cells to glow when it
integrates into the genome (I refer you back to the previous post for details
of the HIV life cycle if you are unfamiliar). They found that this HIV virus
was able to integrate into all three of the different cell types. However, the
cells that were lacking for SLFN11 released a much higher level of new virus
that those that had the protein. This indicates that SLFN11 is applying some
sort of restrictive affect on the HIV life cycle between integration and
release of new virus from the cell. (The removal of SLFN5 had no affect so will
not be mentioned again).
To
only show that removal of SLFN11 allows more virus to be released does not give
a full picture, so the team set out to further confirm the results that SLFN11
is applying a block to the HIV life cycle. To do this they went the other way,
instead of removing the SLFN11 protein from cells that usually have it, they added
it to cells that do not usually have it (the 293T cells). Addition of SLFN11 to
293T cells caused a substantial reduction in the level of virus released from
the cells and this reduction was of a similar magnitude to that seen in the 293
cells that were naturally expressing the protein. This gives two lines of
evidence that SLFN11 is indeed causing a block to the HIV life cycle after
integration.
There
are multiple steps between HIV DNA becoming integrated to the host and the
release of viral particles. In the interest of developing a full understanding
of the role SLFN11 is playing the team began to zoom in on exactly where the
protein was having its restrictive affect. They took the logical approach of
looking at the next step after integration. Once HIV DNA is integrated to the
host genome it is used to make proteins via an intermediate of RNA. This is how
all proteins are made; DNA acts as a template to make RNA that is then used as
a code to make protein. The team looked to see if there was any difference in
the level of HIV RNA produced in the cells that expressed SLFN11 compared to
those that don’t. Unlike the results seen for release of new virus, the
presence of SLFN11 had no affect on the levels of HIV RNA seen inside the cell.
The window in which SLFN11 causes a block is narrowing, we now know the block
occurs between production of RNA and release from the cell.
Having
ruled out the first step post-integration the team moved on to look at the
final step of the HIV life cycle; release from the cell. As I mentioned in the
previous post, the protein tetherin acts at this final stage and causes
accumulation of virus particles on the cell. The team looked for similar
affects in the SLFN11 expressing cells and saw none. We now have evidence that
the block is occurring between production of RNA and before the virus starts to
leave the cell. This pretty much leaves two stages, production of proteins and assembly
of these proteins into new virus particles.
Knowing
that SLFN11 was either affecting protein expression or assembly of the virus
the team looked for the presence of viral proteins inside the cells. They found
that there was substantially less viral protein inside the cells that expressed
SLFN11 even though there was the same amount of RNA from which the protein is
made. The really interesting thing about this is that human proteins being expressed
in the cells were completely unaffected. This means SLFN11 is causing a block
to viral protein expression but not host protein expression; the protein is
therefore highly specific to the invading pathogen. The team further confirmed
this finding by adding artificial DNA to a cell that coded for a viral protein
or a non-viral protein and saw that only the viral protein was blocked for
expression. The evidence with both actual virus and with artificial DNA
strongly supports the idea that SLFN11 is blocking viral proteins from being
produced, and is doing so in a specific manner.
The
question then becomes why. Why is it that SLFN11 only affects viral protein
production? What is different between the RNA of the virus that makes protein
compared to the human RNA that makes protein? For me to answer this question I
need to tell you a small bit about DNA.
A molecule of DNA is made up of chemicals known as nucleotides of which
there are four, known as A, T, C and G. In the DNA double helix, A always binds
to T while C always binds to G. It has been observed that viruses and humans
have different biases in the nucleotide composition of their genomes. Humans have
a bias towards GC nucleotides (around 60%) in our DNA, while certain viruses have
a bias towards AT nucleotides (I will however refer to the viruses as low GC,
though this is obviously the same as high AT).
 |
| An illustration of DNA. I have mentioned A, T, C and G which is the short hand for the chemicals listed in this image. |
Knowing
of the difference in nucleotide bias between humans and viruses, the team asked
whether SLFN11 was taking advantage of this. To address this question they
produced two artificial DNA constructs for a HIV protein known as gag. One of these constructs used the
normal sequence for gag (low GC
content) while the other construct changed all the nucleotides that could be
changed, without affecting the protein, to C or G, so to shift the bias towards
that of a human gene (a gag gene that
is high GC). They found that expression of the normal gag construct was inhibited, as expected, while expression of the high
GC gag gene was not inhibited by
SLFN11 at all. This gives good evidence that SLFN11 is indeed taking advantage
of the differences between the nucleotides used by HIV compared to those used
in humans.
A
further validation of the findings was once again pursued. To achieve this the
team took a protein known as enhanced green fluorescence protein (EGFP) that
can be expressed in human cells and, as the name implies, fluoresces green.
When the team changed the EGFP DNA to have low GC (so to make it like a HIV
gene) it was seen that SLFN11 inhibited the levels of fluorescence in the
cells, so is therefore inhibiting EGFP protein production. This evidence
further supports the idea that the SLFN11 protein is specific to viral proteins
as a function of their nucleotide bias, not some other function of their genome.
That
concludes the story… sort of. It still isn’t clear exactly how SLFN11 blocks expression of proteins, but we know that
it does, and we know why it is highly specific to viral proteins. Hopefully this
post hasn’t been too hard to follow for any non-scientists reading, I’ve tried
my best to explain without making the post overly long. I appreciate that I may
have skipped over some parts particularly surrounding expression of proteins,
so if anything is unclear please feel free to leave a comment and ask a
question. I also hope I have managed to convey what I perceive as the beautifully
logical progression of this paper. The team found that their protein did not
stop HIV integration but did stop release of new viruses. They then looked to
zoom in on exactly where this block was occurring by showing that RNA
production and release of virus were not altered. This led them to look at the
production of proteins where they found a substantial block in the presence of
SLFN11. The question then became why was SLFN11 specifically blocking viral proteins
but not human proteins. A question answered by looking at the different bias of
human and viral DNA for G and C nucleotides. Hopefully it also shows how
science relies on negative and positive confirmations of findings, for
instance, when no SLFN11 then observe more virus release (negative), while the
addition of SLFN11 to these cells causes a block (positive), giving very nice
proof to the role of SLFN11.
Not
only is the discovery of SLFN11 very interesting in regards to HIV but since it
targets all genetic material that has a bias towards low GC content (as shown
with the EGFP experiment) this implies it would be able to target other
viruses. One such virus with a similar bias as HIV is influenza. If we can make
drugs that act in a similar way we may well be able to make broadly acting
anti-viral drugs that are highly specific to virally infected cells, helping to
reduce their side affects and improve their efficacy.
The
fact that SLFN11 may have the potential to act on influenza leads me nicely
into the topic of my next post, the protein IFITM3. Make sure you come back for
that if you’re interested.
you should talk about the effects of fever/hyperthermia on HIV-1
ReplyDeletean interesting read :)
Maybe one for the future. Think I would need to do some background reading first. And thanks :)
Delete